Abstract
Introduction
Although many new therapeutic agents have been introduced in the field of AML, the most important intensive induction regimen of AML patients is still 7+3 chemotherapy based on cytarabine and anthracycline. Currently, major guidelines recommend to exam bone marrow (BM) assessment in 14-21 days after the initiation of induction to determine whether to proceed with intensification chemotherapy. However, there is no solid evidence that these timings are most optimal. If the evaluation at an earlier time point is prognostic for response, earlier intensification could be considered. In this regard, we investigated if the BM blast rate at the 7th day after the start of 7+3 chemotherapy (D7 BM blast) was useful in predicting the treatment response of induction chemotherapy.
Methods
We retrospectively collected the data of patients who were newly diagnosed with AML from February 2002 to February 2021, received induction chemotherapy by 7+3, had a D7 BM examination without any intensification. A total of 665 patients were enrolled and we analyzed the prognostic significance of the D7 BM blast for the induction treatment response (complete remission or complete remission with incomplete hematologic recovery (CR/CRi)). In addition, we analyzed whether the predictive significance of the D7 BM blast varies by the patient's cytogenetic features by Medical Research Council classification (MRC risk). Then, we evaluated the diagnostic ability of the D7 BM blast by the receiver operating characteristic (ROC) curve for treatment response prediction. To find an optimal D7 BM blast cut-off value, the value that maximizes Youden's index was investigated.
Results
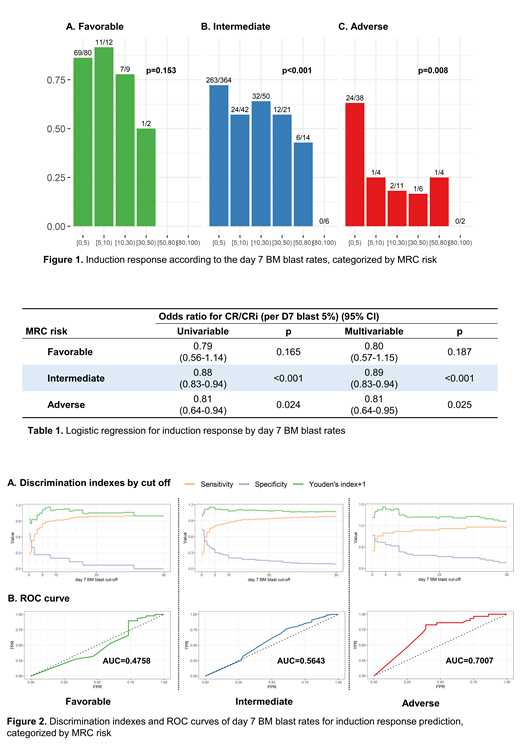
Among 665 AML patients who underwent 7+3 without intensification, the proportion of patients who acquired CR/CRi after single induction was 68.3%. A significant decrease in the CR/CRi rate was observed in the intermediate/adverse MRC group according to the increase of the D7 BM blast (tests for the trends in the intermediate and adverse group, p<0.001 and p=0.008, respectively; Figure 1). In univariable/multivariable (using covariates of age, sex, etiology, and MRC risk) logistic regression models, the D7 BM blast showed a significant correlation with the CR/CRi rate in the intermediate/adverse cytogenetic group (Table 1). To evaluate the usability of the D7 BM blast as a predictive tool, the ROC curve for the treatment response prediction was plotted (Figure 2) and the D7 BM blast was significantly predictable only in the adverse MRC risk group (Area under the curve: 0.7007, Mann-Whitney test statistics p=0.002). The D7 blast cut-off in the adverse MRC risk group which maximizes Youden's index was 4-4.9%, near to 5%, which is the cut-off used to evaluate the treatment response. The sensitivity and specificity for treatment response prediction according to D7 BM blast <5% or not were 82.8% and 61.1%, respectively.
Conclusion
The BM assessment performed at 7 days after 7+3 chemotherapy was available to predict the treatment response in intermediate and adverse cytogenetic risk patients. In particular, it can be practically used in patients with adverse cytogenetics, and a value of around BM blast 5% can be used as cut-off for response prediction. Early intensification in these patients could be considered, which would be beneficial in various aspects such as shorter nadir period or hospital stay and possibly better treatment response compared with the current strategies of later intensification or second induction.
Kim: Novartis: Research Funding; BMS: Research Funding; Pfizer: Research Funding; ILYANG: Research Funding; Takeda: Research Funding. Lee: Alexion, AstraZeneca Rare Disease: Honoraria, Membership on an entity's Board of Directors or advisory committees. Kim: AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; AIMS Biosciense: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; AML-Hub: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; BL & H: Research Funding; BMS & Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Boryung Pharm Co.: Consultancy; Daiichi Sankyo: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Handok: Consultancy, Honoraria; LG Chem: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Honoraria; Pintherapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sanofi Genzyme: Honoraria, Speakers Bureau; SL VaxiGen: Consultancy, Honoraria; VigenCell: Consultancy, Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal